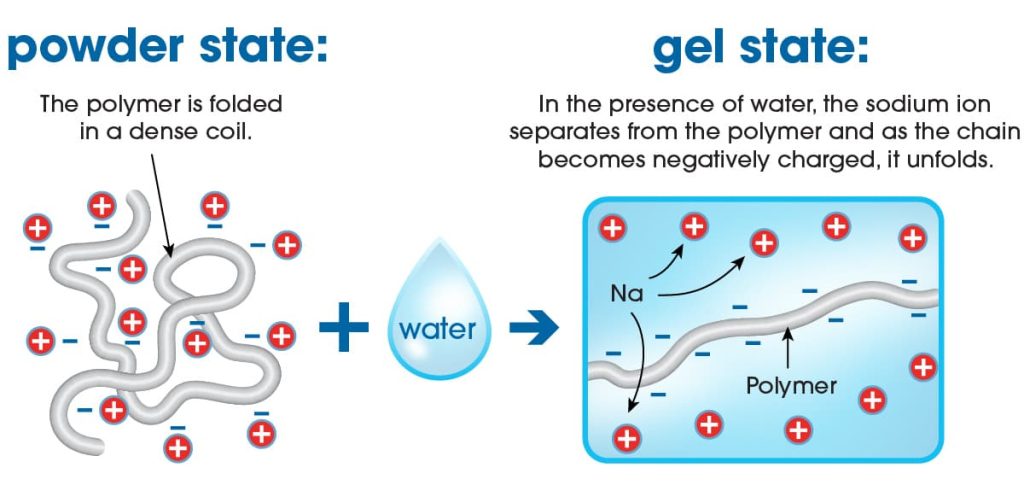
Sodium acrylate and sodium polyacrylate are related compounds but have distinct differences:
- Chemical Structure:
- Sodium Acrylate: This is a salt of acrylic acid, specifically the sodium salt. Its chemical formula is CH2=CHCOONa. It is a monomer, meaning it consists of a single repeating unit.
- Sodium Polyacrylate: This is a polymer derived from acrylic acid. It consists of multiple repeating units of sodium acrylate linked together through chemical bonds. Its chemical formula can be represented as (CH2=CHCOONa)n, indicating a chain of repeated sodium acrylate units.
- Physical Properties:
- Sodium Acrylate: As a monomer, sodium acrylate is typically in the form of a white crystalline powder. It is soluble in water and has various industrial uses, such as in the production of superabsorbent polymers.
- Sodium Polyacrylate: This polymer is usually in the form of a fine powder or granules. It has a high water-absorbing capacity, making it suitable for use in products like diapers, feminine hygiene products, and agricultural soil amendments.
- Applications:
- Sodium Acrylate: It is primarily used as a precursor in the synthesis of sodium polyacrylate, which is the superabsorbent polymer widely used in absorbent products.
- Sodium Polyacrylate: This polymer is used extensively in industries where moisture absorption and retention are critical, such as in hygiene products, agriculture, and certain medical applications.
In summary, sodium acrylate is the monomeric form of acrylic acid’s sodium salt, while sodium polyacrylate is a polymer made from multiple units of sodium acrylate linked together, known for its superabsorbent properties.

